0102030405
Urolithin A Shows Promise in Treating Alzheimer’s Disease
2024-12-26
Recently, the team of Professor Hou Yujun from the School of Life Science and Technology of Tongji University/Tongji University Affiliated Dongfang Hospital published the latest research results, which found that long-term Urolithin A (UA) treatment significantly improved the learning, memory and olfactory functions of AD mice, and reduced Aβ and tau pathology.
Further research found that UA induces mitophagy by enhancing lysosomal function. UA treatment can normalize lysosomal cathepsins, especially cathepsin z, thereby restoring lysosomal function in AD mice.
Professor Vilhelm Bohr of the National Institutes of Health is the co-corresponding author. The study was published in Alzheimer’s & Dementia.

Impairment of mitophagy may have occurred before the main pathology of AD occurs. Therefore, early intervention in mitophagy is very important. APP/PS1 mice generally develop Aβ pathology and behavioral defects at 6 months of age. Therefore, the researchers exposed 2-month-old APP/PS1 mice to UA for 5 months, and then evaluated the behavioral performance and behavior of the mice. Brain samples are tested.
The results of the water maze experiment showed that UA treatment successfully improved the memory and learning abilities of APP/PS1 mice, and the performance of the mice was basically the same as that of wild-type mice. When tested again one month after stopping the drug, the UA-treated mice still showed better memory ability than the APP/PS1 mice, demonstrating the lasting impact of UA treatment on AD endpoints.
To better mimic human AD features, the researchers developed a DNA repair-deficient ADP mouse model, which typically develops Aβ and tau pathology around 12 months of age. Oral administration of UA started in ADP mice at 12 months of age and continued for 5 months. Significant improvements in memory and cognitive abilities of ADP mice were observed.
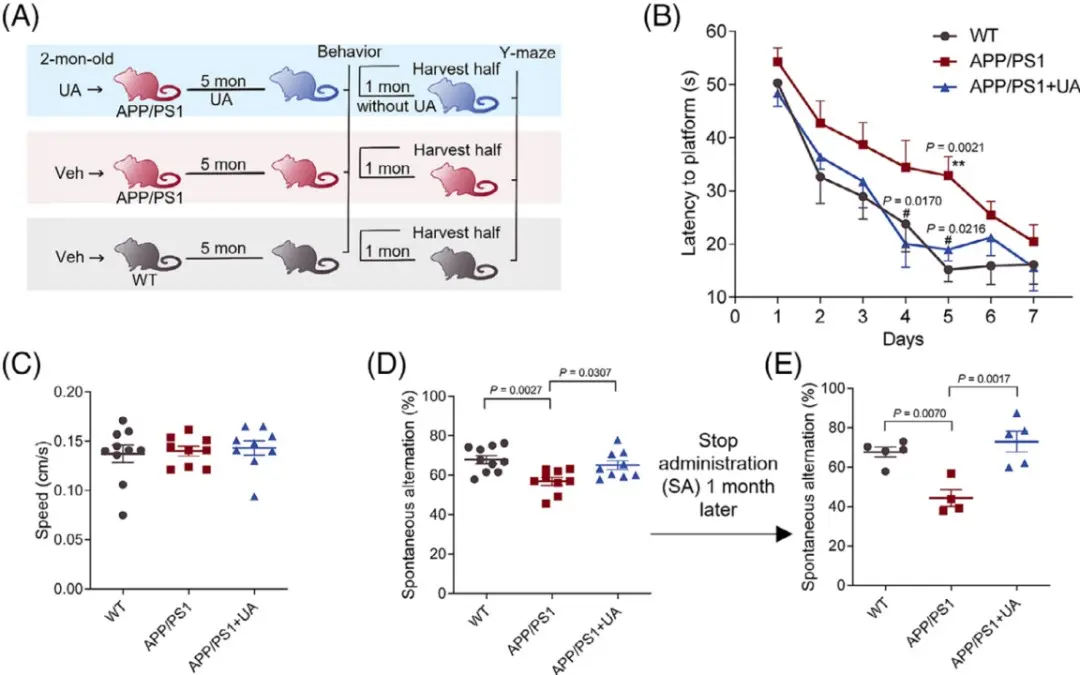
Figure: Long-term UA treatment improves learning and memory abilities in AD mice
The olfactory impairment that often occurs in AD patients was also significantly improved after long-term UA treatment, and the mice no longer showed obvious olfactory impairment. Electrophysiological experimental studies showed that the impaired synaptic function of ADP mice was also significantly improved.
Further observations revealed that after UA treatment, Aβ deposition levels in the prefrontal cortex of APP/PS1 mice were reduced, the number of abnormally activated microglia in the prefrontal cortex and hippocampus was smaller, and p-tau levels in the brains of ADP mice were reduced.
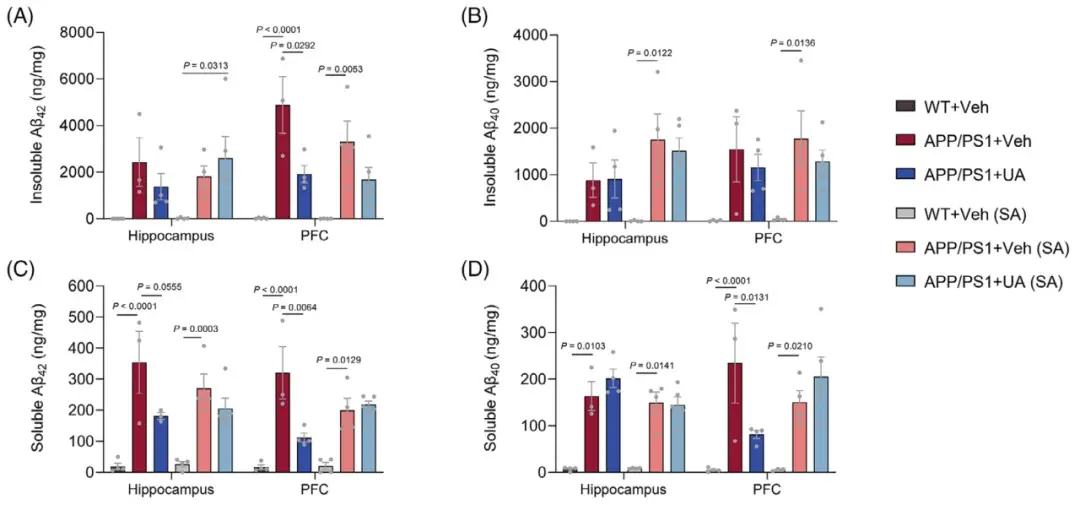
Figure: UA treatment reduces Aβ deposition levels in AD mice
In order to explore the potential mechanism of UA in treating AD, the researchers conducted a microarray analysis of gene expression in the hippocampus tissue of APP/PS1 mice and found that compared with wild-type mice, there were significant changes in lysosome-related genes, among which tissue Proteinase z (Ctsz) was significantly upregulated in APP/PS1 mice and returned to wild-type levels after UA treatment. The lysosome sensor test showed that UA treatment significantly improved lysosomal function and promoted lysosomal acidification, and knocking down Ctsz expression affected the UA-induced improvement of lysosomal function.
In addition to changes in lysosomes, researchers also found that UA regulates the expression of specific inflammatory factors (IL-1β, IL-10) and reduces the expression of pro-inflammatory cytokines and inflammatory signaling pathways (such as NF-κB, AIM2, pSTAT3/STAT3 , and improve mitochondrial function by increasing the expression of mitophagy-related proteins, and also help reduce DNA damage.
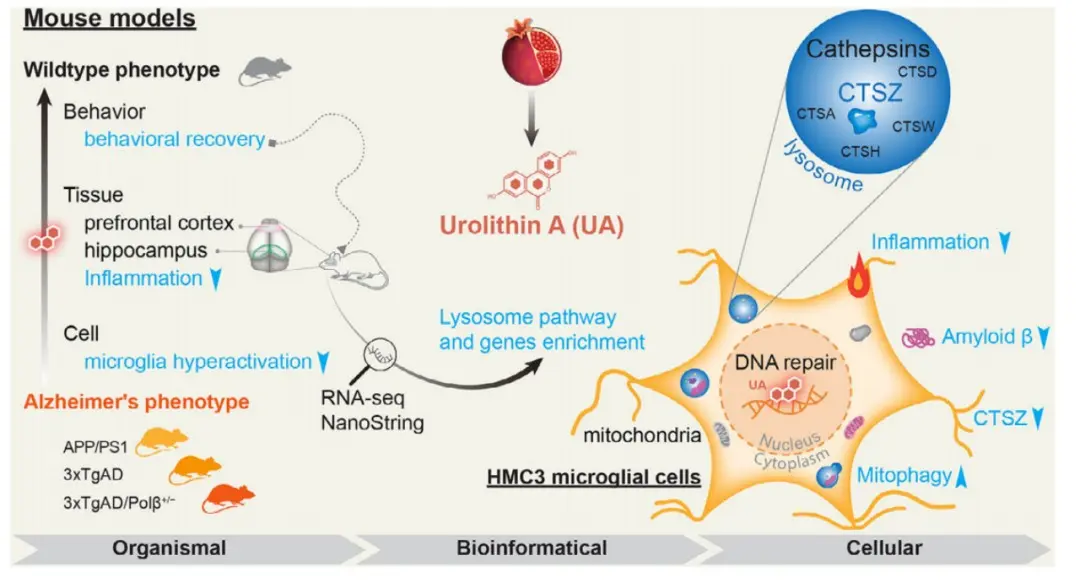
Figure: Mechanisms of UA improving AD symptoms
Overall, the study identified the potential mechanism of medium- and long-term UA treatment for AD, involving multiple neuropathologies in the hippocampus and prefrontal cortex regions, and improving synaptic function, Aβ deposition, p-tau, neuroinflammation, lysosomal function, and DNA damage. The study also demonstrated for the first time the important role of Ctsz in UA treatment of AD.
The study emphasized the importance of lysosomal dysfunction in the pathogenesis of AD and showed the development prospects of Urolithin A in the treatment of AD.