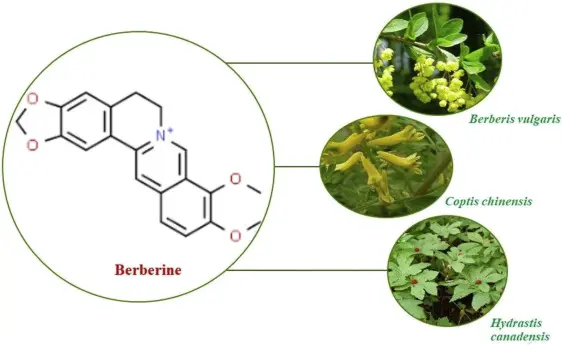
Self-Assembled GA-BBR Herbal Hydrogel Breakthrough for Ulcerative Colitis Treatment
On December 9, 2024, a research team led by Professors Li Chao and Qiao Renzhong from the College of Life Science and Technology at Beijing University of Chemical Technology published an innovative study in the Chemical Engineering Journal (IF 13.4). The article, titled "Self-assembled herbal hydrogel for rectal administration therapy in ulcerative colitis", presents a groundbreaking carrier-free hydrogel formed through the self-assembly of berberine (BBR) and glycyrrhizic acid (GA). This novel treatment targets ulcerative colitis (UC) using rectal administration and offers a safe, cost-effective, and efficient therapeutic approach.
Key Findings
1. Development of GA-BBR Carrier-Free Hydrogel
The team successfully designed and synthesized a GA-BBR hydrogel through precise self-assembly. Their experiments demonstrated that the ratio of glycyrrhizic acid to Berberine in the hydrogel closely matched the initial feed ratio, with a consistent self-assembly ratio of 3:1 when the feed ratio was set accordingly. This precise formulation provides a solid foundation for future research and clinical applications.
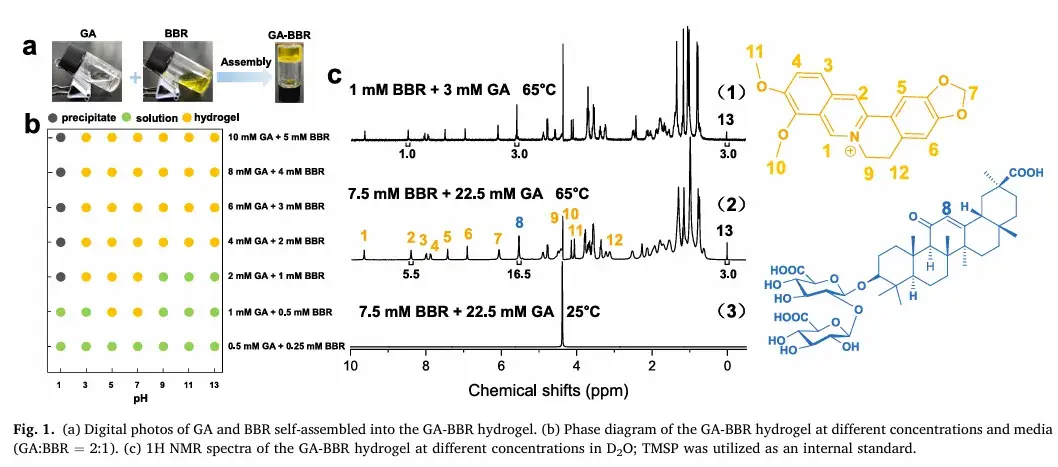
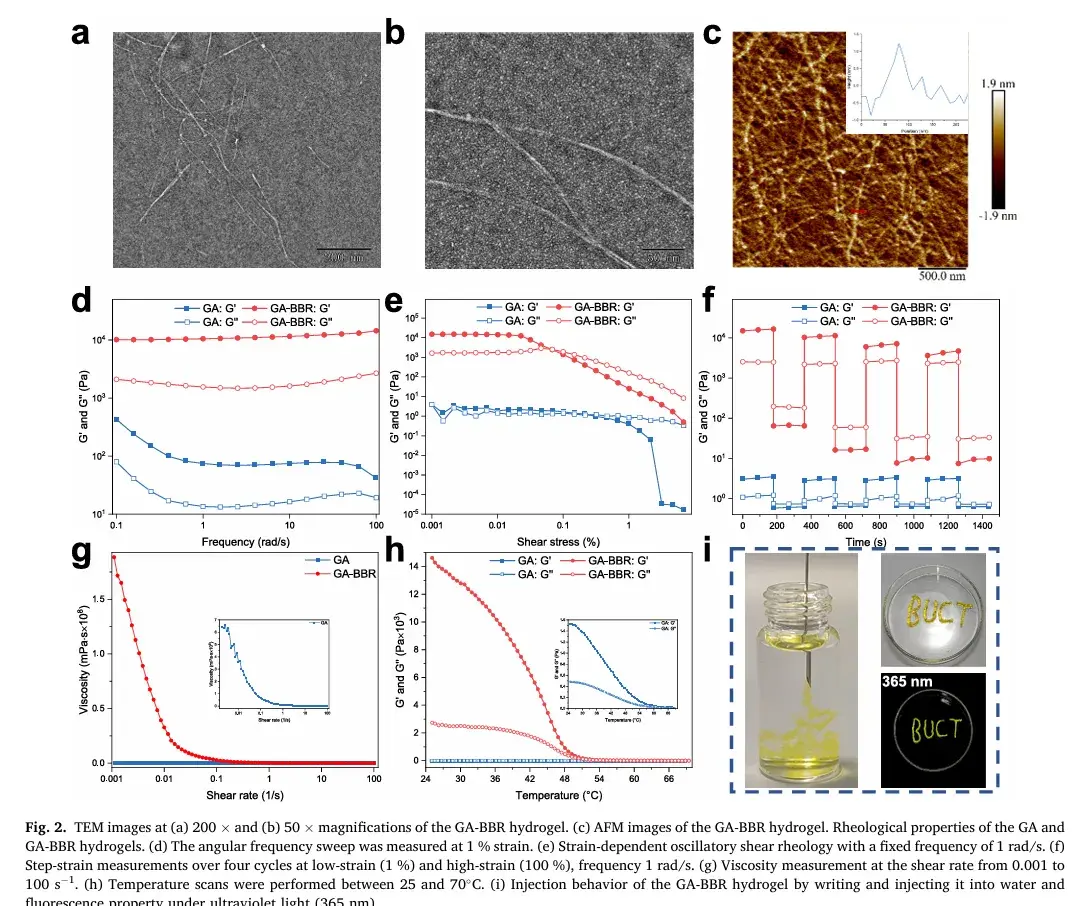
2. Morphological and Rheological Properties
Comprehensive tests revealed the hydrogel’s distinct nanofiber structure with a diameter of 50 nm and a height of 2 nm. Rheological measurements and skin tests further confirmed its excellent injectability and adhesive properties, making it ideal for rectal administration as a localized treatment for UC.
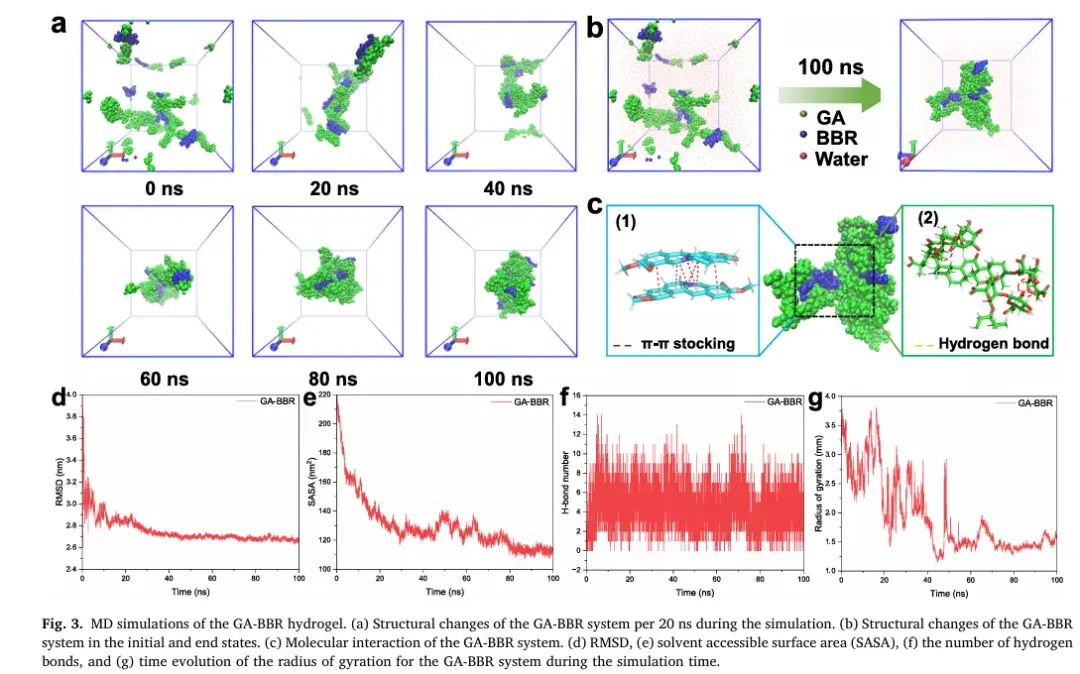
3. Molecular Dynamics and Self-Assembly Mechanism
Molecular dynamics (MD) simulations were employed to explore the self-assembly process in detail. The results, corroborated by experimental validation, highlighted the critical roles of hydrogen bonding, π-π stacking, and electrostatic interactions in the formation of the hydrogel. These findings provide valuable insights into the underlying mechanisms of GA-BBR hydrogel self-assembly.
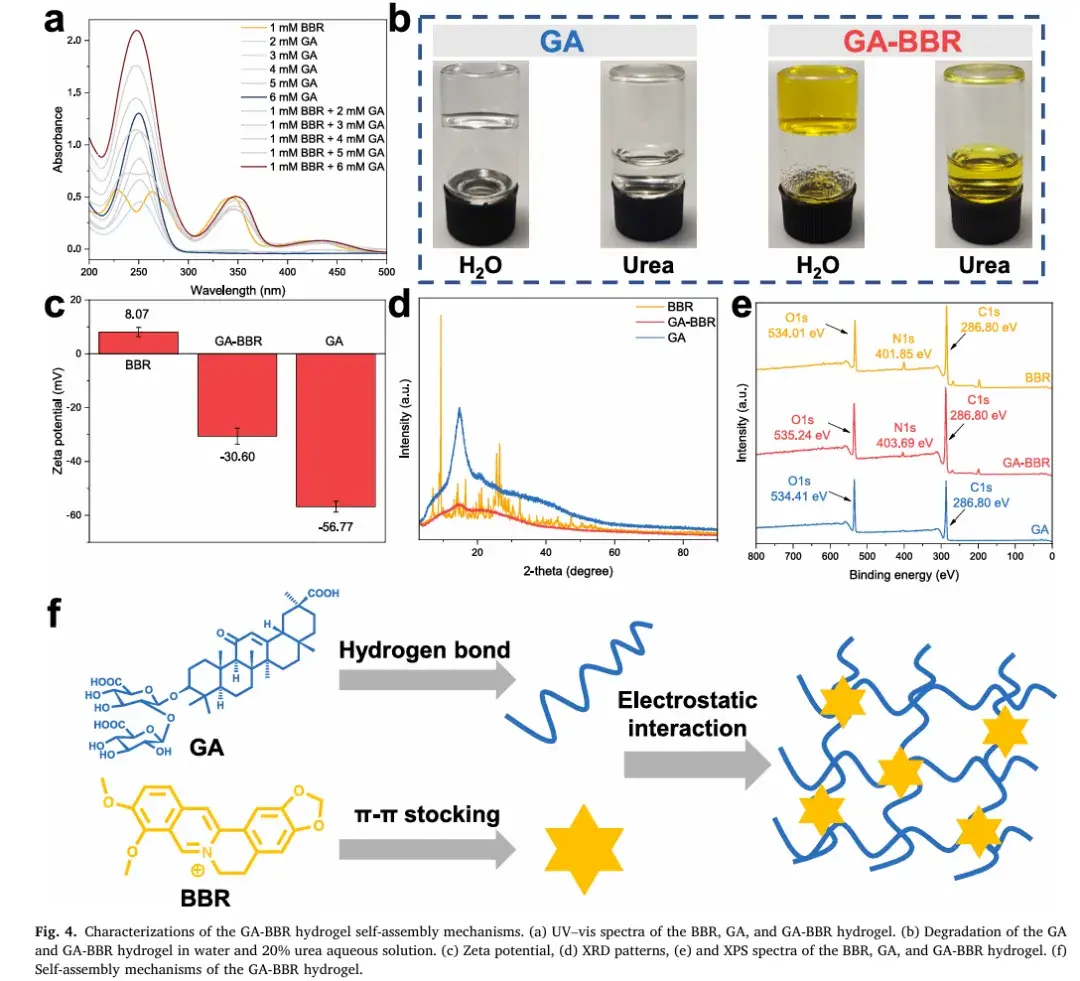
4. Fluorescence Enhancement of Berberine
Interestingly, the study discovered that GA significantly enhances the fluorescence intensity of Berberine. Under UV light at 365 nm, the weak fluorescence emitted by pure Berberine became significantly brighter within the GA-BBR hydrogel. This fluorescence enhancement, attributed to an aggregation-induced emission (AIE) mechanism, opens new avenues for Berberine’s application in fluorescence-based technologies.
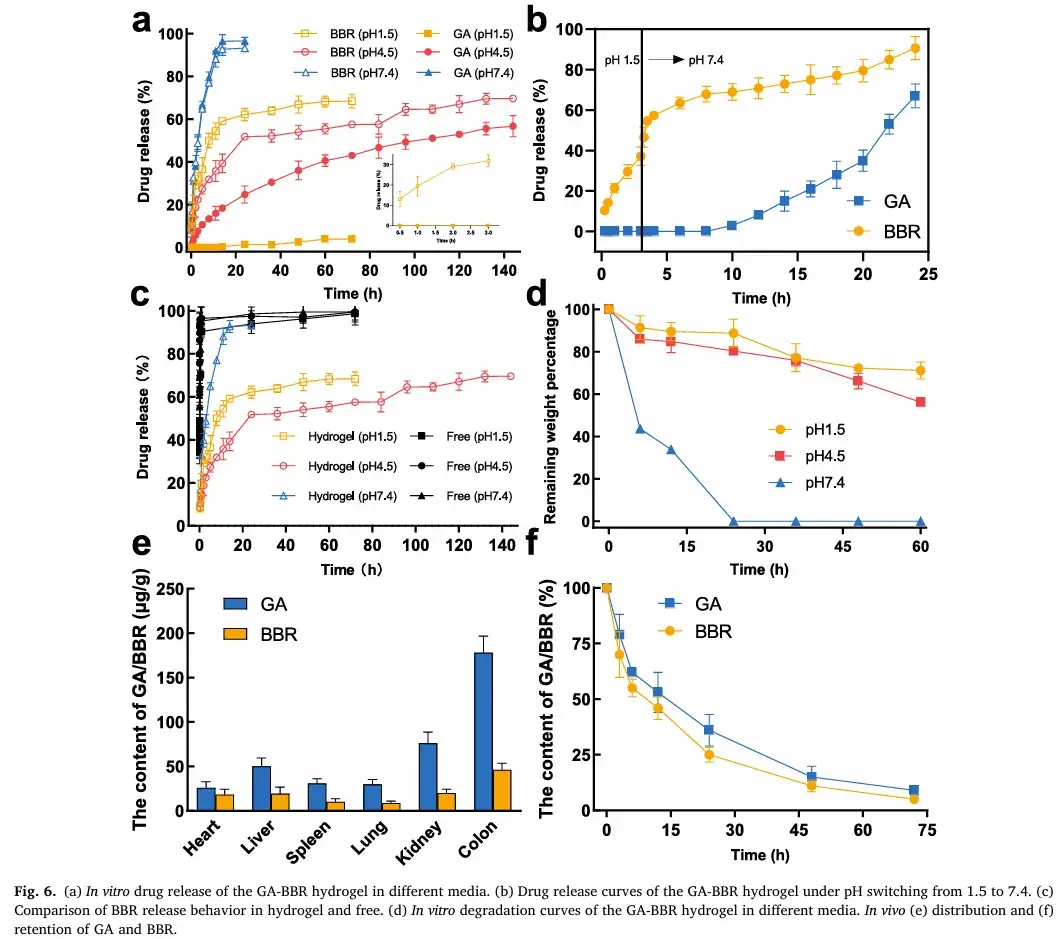
5. Drug Release and Biodegradability
The GA-BBR hydrogel demonstrated outstanding drug release behavior, safety, and acid resistance. Under pH 4.5 conditions, it sustained drug release for up to 140 hours, ensuring prolonged therapeutic effects. Its degradation properties and minimal cytotoxicity further confirm its safety for clinical use, particularly in treating moderate to severe UC.
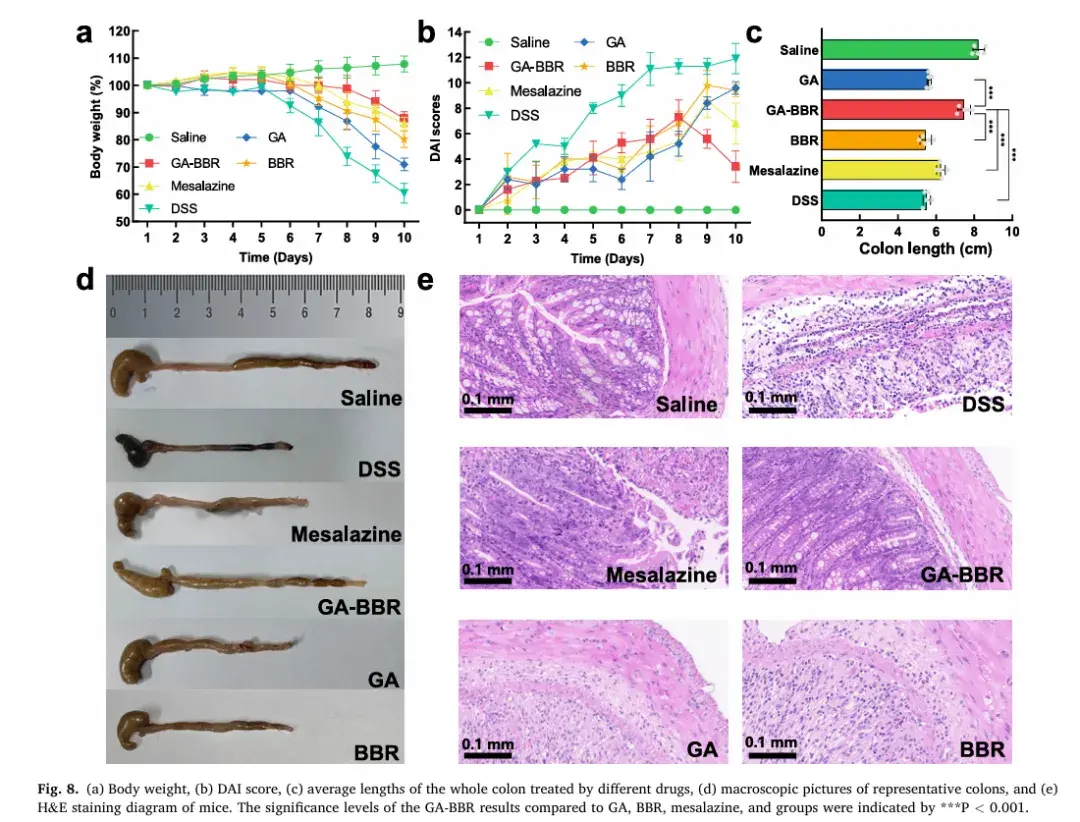
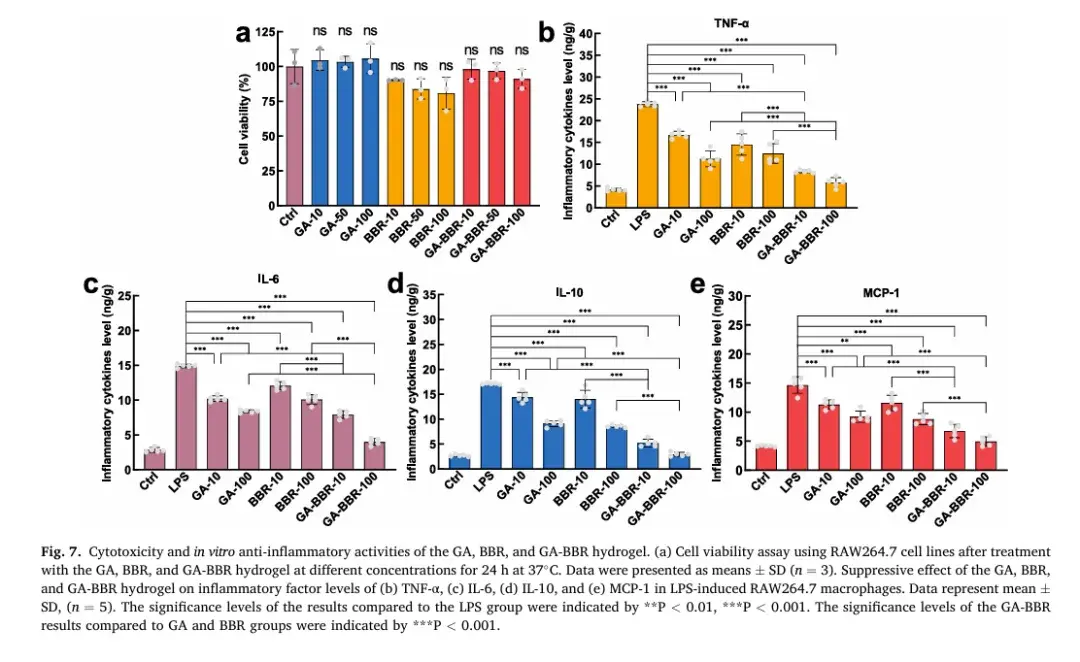
6. Anti-inflammatory Activity In Vitro and In Vivo
Both in vitro and in vivo experiments showed the hydrogel’s superior anti-inflammatory effects compared to mesalazine, GA, or BBR monotherapy. The GA-BBR hydrogel significantly improved key indicators of UC, such as body weight, disease activity index (DAI) score, colon length, histological changes, and inflammation levels. These findings highlight its potential as a novel and highly effective treatment for UC.
Implications for Ulcerative Colitis Treatment
This study introduces an innovative approach to UC management through a carrier-free hydrogel composed of natural compounds. By combining the therapeutic effects of Glycyrrhizic acid and Berberine, the hydrogel delivers sustained, localized treatment with minimal side effects. Its dual functionality—anti-inflammatory action and controlled drug release—represents a significant advancement in UC therapy.
The GA-BBR hydrogel sets a new benchmark for cost-effective and efficient treatment strategies for inflammatory diseases. Future research may expand its application to other conditions, paving the way for broader clinical use.